Fewer Steps Are Required to Solve Stoichiometry Problems When
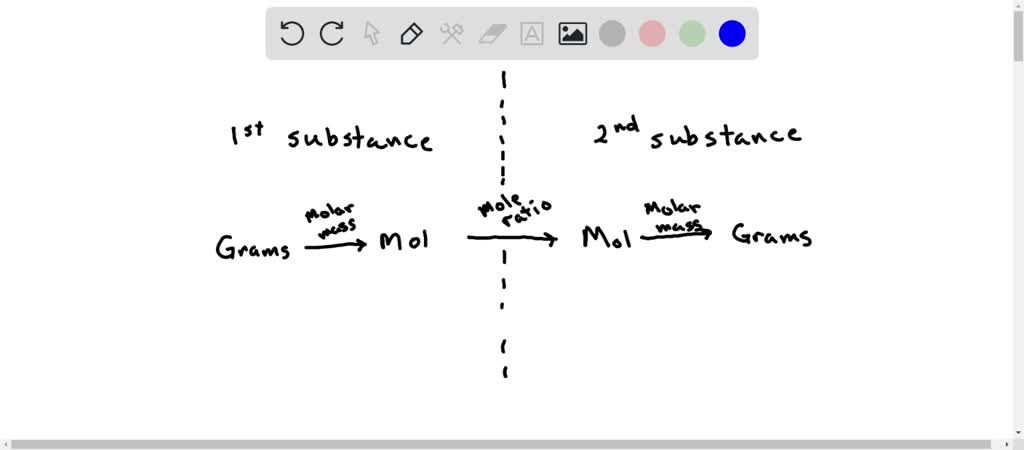
There are four steps in solving a stoichiometry problem. Want this question answered.

Moles Activity Maze Teaching Chemistry Middle School Science Experiments Chemistry
A the amount of reactants given in grams and the product is sought in grams.

. Mechanism involved in the reaction. What volume how much solution. This rarely happens in the lab or in industry o YETtheoretical calculations are important because they show the.
A the reactant is given in grams and the product is sought in grams. Start with your balanced chemical equation. C 6 H 12 O 6 2C 2 H 5 OH 2CO 2.
Fewer steps are required to solve stoichiometry problems when the reactant is given in moles and the product is sought in moles The participation of reactants in a chemical reaction is restricted by the. C the amount reactant is. How many grams of water can be prepared.
Write the balanced chemical equation. Posted by Melissa Valqui. Knowledge of the problem solver Hayes 1981.
Balance the equation 2. For this chapter we assume that the amounts of reactants and products for a given reaction occur under ideal conditions. C the reactant is given in grams and the product is sought in liters.
Fewer steps are required to solve stoichiometry problems when Question options. Be notified when an answer is. So what you want to do is you want to go from grams to moles and then once youre in moles you can go to moles off anything else in the chemical equation using the mole ratio.
Solve for M. B the reactant is given in moles and the product is sought in moles. New questions in English given below are the two most common bills at home compare them by completing the table.
Remember to solve ANY stoichiometry problem. Use the mole ratio to calculate the moles of wanted substance B. All reactants are completely converted into products.
So in terms of my quick three easy steps because youre very rarely gonna get a problem. The amount of reactant is given in grams and the product is sought in grams. The amount of reactant is given in moles and the product is sought in moles.
In this video we will look at the steps to solving stoichiometry problems. Question 2 60 seconds Report an issue Q. 400 This is the unit for measuring the mass of an atom.
NAME OF MATERIAL SOURCE HOW OFTEN YOUR FAMILY. 1 pts Question 4 Fewer steps are required to solve stoichiometry problems when the amounts of reactants are given in moles the amounts of reactants are given in grams. Where there giving They give you the number of moles of your reactant.
Convert the given mass or number of particles of a substance to the number of moles. Convert moles of wanted substance to desired units EXAMPLE. English Junior High School Fewer steps are required to solve stoichiometry problems when elizabethalmosa8715 is waiting for your help.
Fewer steps are required to solve stoichiometry problems when. A gram to gram stoichiometry problem must include answer choices a gram to mole conversion factor a mole to gram conversion factor a gram to mole a mole to gram conversion factor. Hydrogen gas combines with oxygen gas to form water.
Question 7 300 seconds Q. Two gram to mole conversion factors. Energy released in the reaction.
Chemistry questions and answers. What concentration what molarity. Fewer steps are required to solve stoichiometry problems when the reactant is given in this unit.
The flow chart below summarizes the process. The reactant is given in moles and the product is sought in. Electron configuration of all elements in the reaction.
In this study the focus was on topic-specific. What is the number of moles. Question 1 60 seconds Report an issue Q.
Add your answer and earn points. What Fewer steps are required to solve stoichiometry problems when. 04 Aug Core Concepts.
Fewer steps are required to solve stoichiometry problems when. Mole ratio of any two substances in the reaction Fewer steps are required to solve stoichiometry problems when the reactant is give in Moles and the product is sought in moles All of the following values can be used to solve stoichiometry calculations EXCEPT Temperature In the reaction N2 3H2 2NH3 what is the mole ratio of ammonia to hydrogen. Determine the mole ratios of the reactants and products how many moles.
Convert moles of the wanted substance to the desired units. Find moles of wanted substance using mole ratio 4. Using dimensional analysis you can use the stoichiometric ratio to find the solution to this.
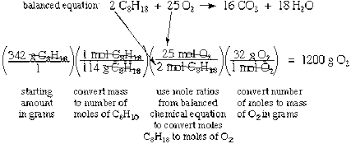
Mass HCl1mol HClmolar mass HCl1 mol Cl₂2 mol HCl In the equation 2Al₂O₃4Al3O₂ what is the mole ratio of aluminum to oxygen. One important component of gasoline mixtures is octane. Fewer steps are required to solve stoichiometry problems when.
B the amount of reactant is given in moles and the product is sought in moles. Solve for n n MV convert moles to grams if necessary. How do you solve a stoichiometry problem.
According to Barnsford and Stein 1984 and Hayes 1981 the five basic approaches to problem solving are working backwards breaking a problem into parts working systematically solving problems by analogy and using procedural and conceptual knowledge. 400 The coefficient represents this in a chemical reaction when solving stoichiometry problems. A balanced chemical equation allows one to determine the answer choices mole ratio of any two substances in the reaction.
Which expression can be used to solve a mass-to-mole conversion for the equation. Calculate number of moles of the required substance based on the number of moles of the given substance using the mole ratio. Use the equation below to solve the problem.
If 25 moles of C 6 H 12 O 6 reacts how many moles of carbon dioxide are produced. FOUR STEPS TO SOLVE STOICHIOMETRIC PROBLEMS 1. Convert units of given substance to moles 3.
H2 g O2 g H2O. Convert the units of the given substance A to moles. The amount reactant is given in grams and the product is sought in liters.
Solve for V V n M. Must begin with a balanced equation. Write a balanced equation 2.

Stoichiometry Assessment Chemistry Quiz Quizizz

Diagramming Galvanic Cells Handout And Worksheet Galvanic Cell Ap Chemistry Biology Worksheet
Solved Fewer Steps Are Required To Solve Stoichiometry Problems When A The Amount Of Reactant Is Given In Grams And The Product Is Sought In Grams B The Amount Of Reactant Is Given
No comments for "Fewer Steps Are Required to Solve Stoichiometry Problems When"
Post a Comment